WASHINGTON — We looked at some of the top questions about the FDA’s approval of the Pfizer vaccine.
Our Sources:
- The Food and Drug Administration. (FDA)
- Dr. William Moss, vaccines expert from the Johns Hopkins University Bloomberg School of Public Health.
- Dr. Anthony Fauci, White Chief Medical Advisor.
Question 1:
What does full approval for the Pfizer vaccine mean?
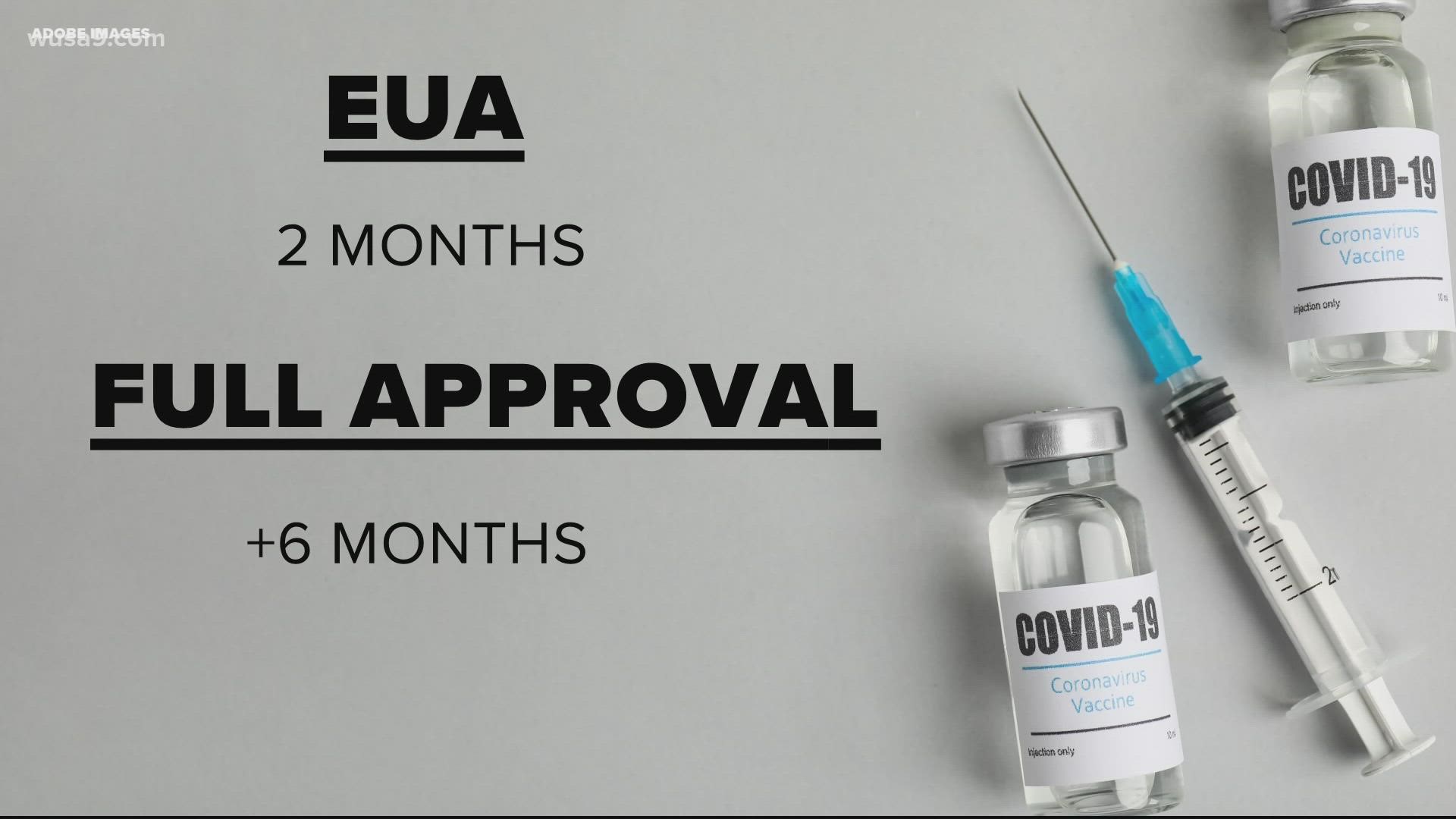
We’ve answered this before. But basically, before the approval, all three vaccines were granted Emergency Use Authorization (EUA) by the FDA. That is an approval used in emergency situations case-in-point, the pandemic.
According to the FDA, the EUA follows the same safety and procedural standards as a full approval.
However, there is a shorter time period and fewer data from the trials required for an EUA compared to full approval.
Question 2:
Why is this just for people 16 years old and up?
Dr. Moss explained the first Pfizer trials were for people 18 and older. The next phase of trials was for 16 and older. Followed by people 12 to 15 years old. The trials for children younger than 12 have just begun.
For full approval, a vaccine needs to show six months of trials and data. The FDA has a rolling acceptance of data from Pfizer. At this point, they are caught up to data from participants 16 and older.
The ages 12 to 15 trials started later, so no full approval, yet. However, there is an Emergency Use Authorization for that age group.
Question 3:
Now that there is full approval, when could kids younger than 12, at least get an Emergency Use Authorization?
In an interview with NPR, Dr. Fauci said he doesn’t know for sure. But he pointed out based on the timelines for Pfizer trials and approvals, kids aged 5 to 11 years old could get an EUA by mid to late Fall.
However, ultimately that decision will be up to the FDA.
Sign up for the Get Up DC newsletter: Your forecast. Your commute. Your news.
Sign up for the Capitol Breach email newsletter, delivering the latest breaking news and a roundup of the investigation into the Capitol Riots on January 6, 2021.