ROCKVILLE, Md. — We are nine months into the pandemic in the U.S. In September, several vaccines entered the final stages of trials. Pharmaceutical companies such as Johnson & Johnson and Moderna have announced they have started Phase 3 testing.
So, who are the people taking part in these studies? And what would make someone want to participate in a clinical trial? We went to find out.
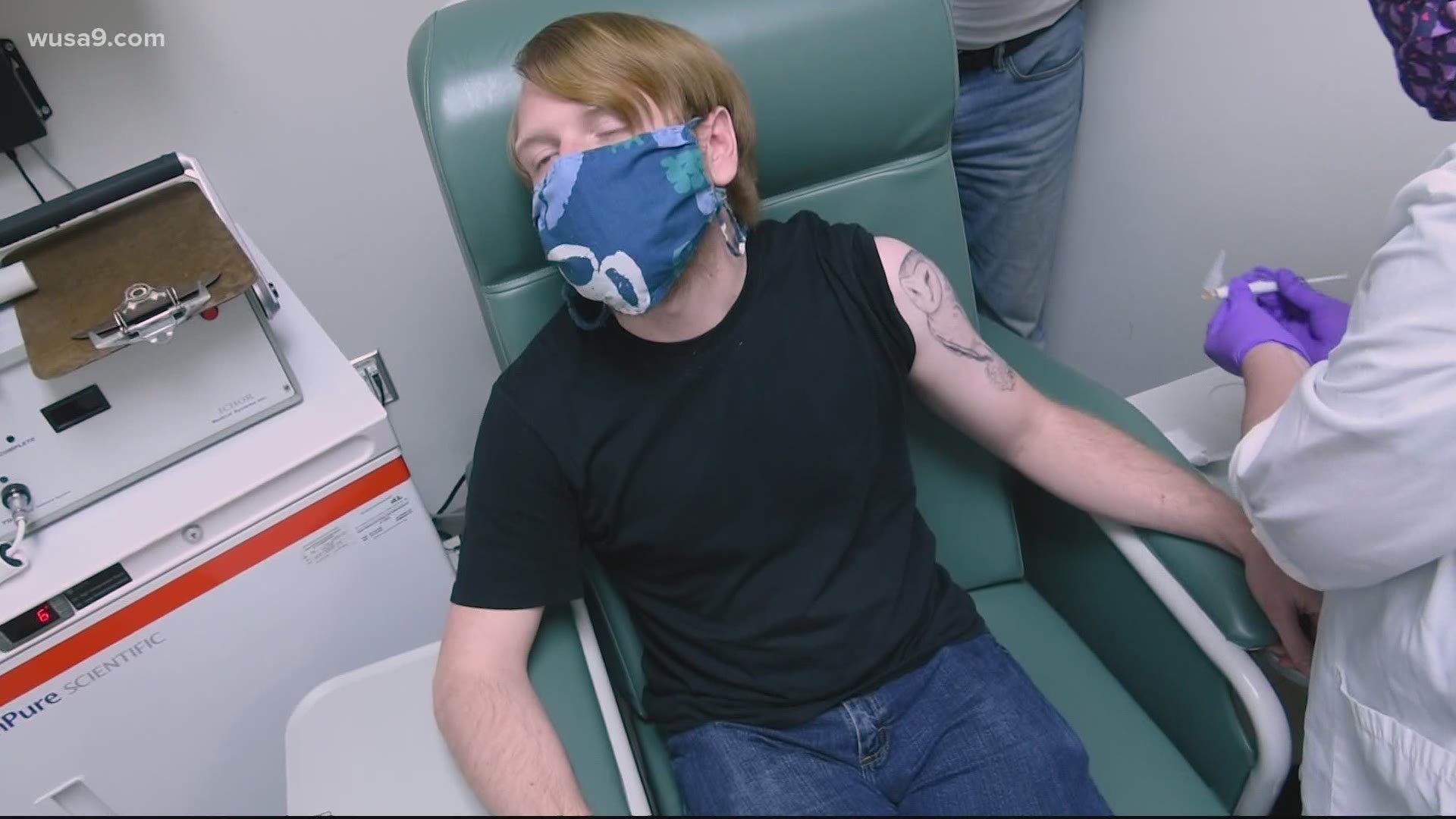
We found Rachel Maness who said she was part of the Moderna trials for a vaccine. We asked if we could follow her at an office park in Rockville, Maryland for her final dose and she agreed. It turns out the battle for our future isn’t being done in a laboratory or a hospital, but nondescript buildings across the country.
If the vaccine trial is the front lines of the battle, then Maness is--one of the soldiers.
“We have every reason to do this to get into the trenches and fight this,” Maness said.
Before she explained the process, we wanted to know why she would agree to be part of an experimental vaccine trial for the coronavirus. Maness explained she did it for people she has never met.
“The idea is someone like me goes out, and I do something to take action to protect you,” she explained. “Then you reciprocate that consideration. After a while, if that builds up, I feel, personally, that's the only way we're going to get out of this mess.”
To pay it forward.
When the pandemic started, she found a few advertisements online to sign up for a vaccine trial. And so she did.
Then a few months ago, she got a call that the Moderna trials were in Phase 3 and they wanted her to come in and be part of the testing.
“I'm ready for my second dose and that I believe, is 28 or so days after your first dose,” she said. “So I'm gonna get that today.”
Here is how vaccine trials go according to the Centers for Disease Control and Prevention (CDC):
Phase 1: A small group receives the trial vaccine.
Phase 2: The study expands to different ages and health ranges to mimic the people that the vaccine is meant to go to.
Phase 3: The vaccine is given to thousands of people for safety purposes and to see how it works.
“I came in, they did intake. I spoke with someone at length. I signed some papers, because it is a trial,” she said.
The Moderna vaccine trial is called a double-blind test. Half of the patients get the vaccine and the other half get a placebo. No one knows who is getting what, not even the people giving out the dose.
“The first night was fine. Second, everything was fine,” Maness said about the first dose. “The second night, I thankfully got a little bit of a temperature. So hopefully, fingers crossed, that was my indication that I didn't receive the placebo.”
But, she doesn’t actually know. Her hope is that her small contribution to the battle against COVID-19 leads to the greater good in the world.
“I'm not gonna sit down and let this happen to my life,” she said. “That's how we should all be, and I really hope that there are people out there who will really start to think about it in those terms and do the same thing. I mean, this is the only way we're going to get through it."