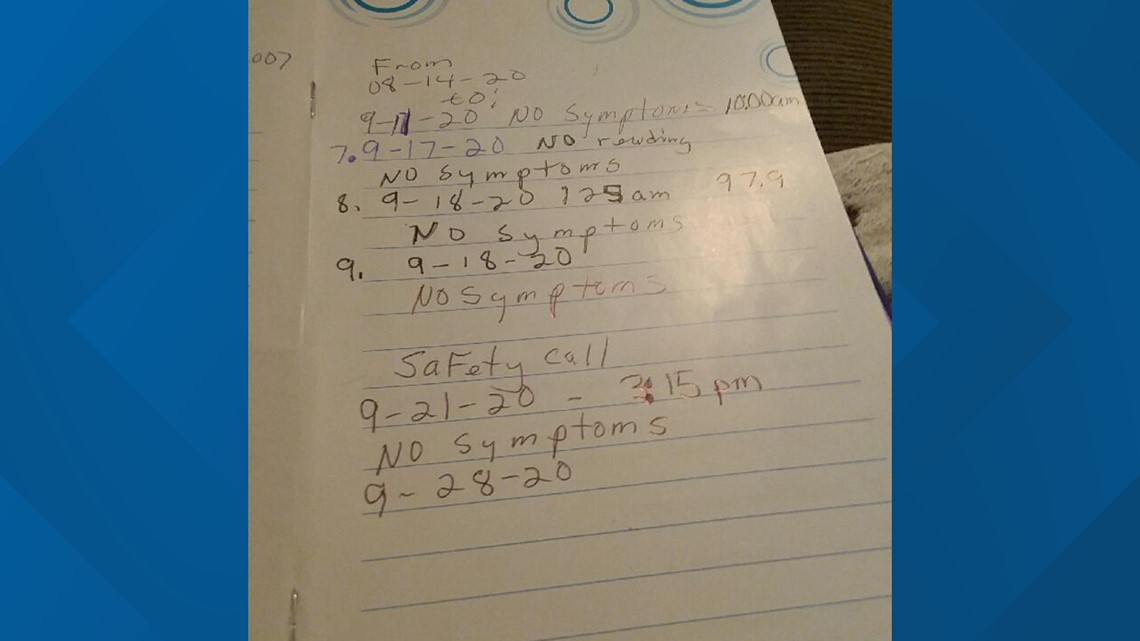WASHINGTON — As the race to develop a safe, effective COVID-19 vaccine continues, a participant who lives in D.C. is sharing her experience as part of the Moderna trial.
Mary Wade is a 68-year-old African American woman who has asthma — falling into three of the CDC's high-risk categories for COVID-19. She's lived in D.C. for nearly 50 years.
When she heard about the vaccine trial at George Washington University Hospital — where she goes to the doctor — she eagerly signed up.
“I’m a strong old lady, and I just want to make a difference in the world," Wade said.
Wade said she had her first injection on August 14 and the second on Sept. 11.
“I’ve been in the study a month, and I’ve had two injections, not knowing if it was a placebo or the actual vaccine," Wade said. "I still have no symptoms.”

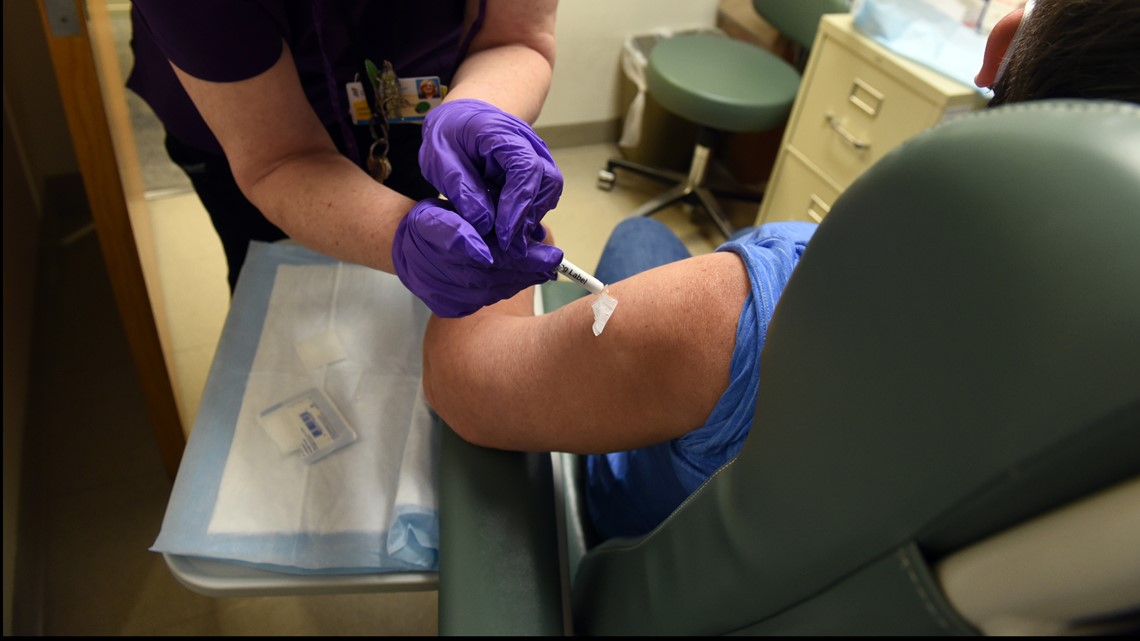
Wade said the process is straightforward, and she felt safe.
“They took about eight vials of blood, and then they do the COVID-19 test," Wade said. "Then they give you a diary … and they follow you immediately after you get home … I started recording for seven days, … putting the date and time and reporting that I had no symptoms.”
Wade has a message for people who do not want to get the vaccine when it's publicly available: "Don’t dwell on fear … because we’re definitely going to need the vaccine.”
Wade said the study team told her she'll be monitored for about the next two years.
Last week, the CEO of Moderna said they expect to have enough data from the phase 3 trial to know in November whether or not their vaccine works and is safe.